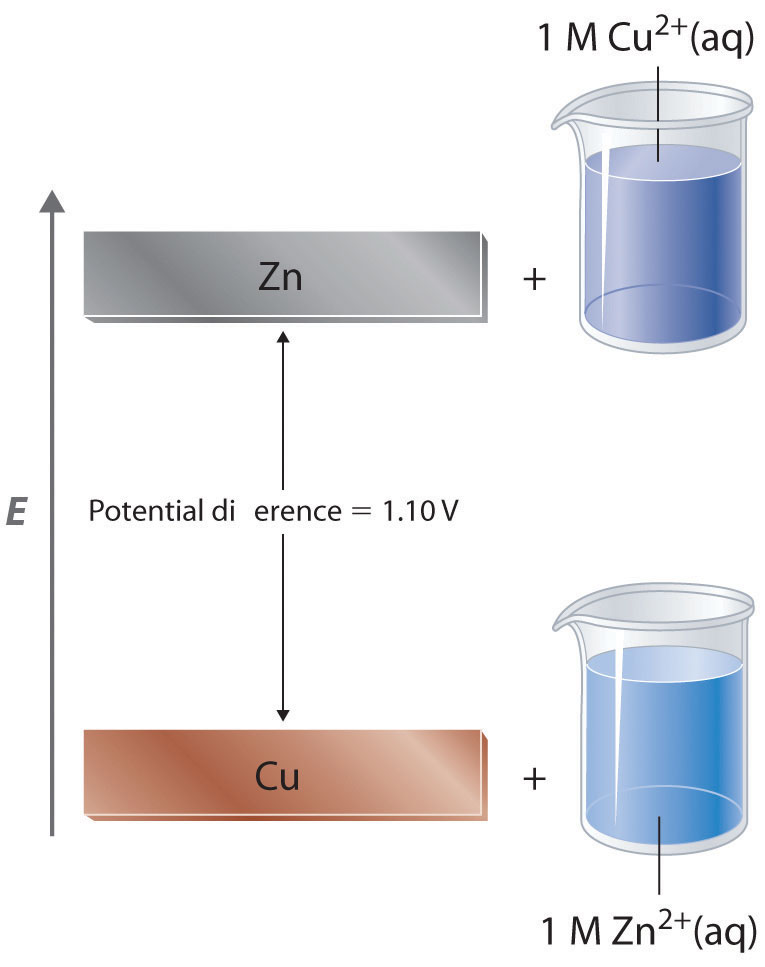
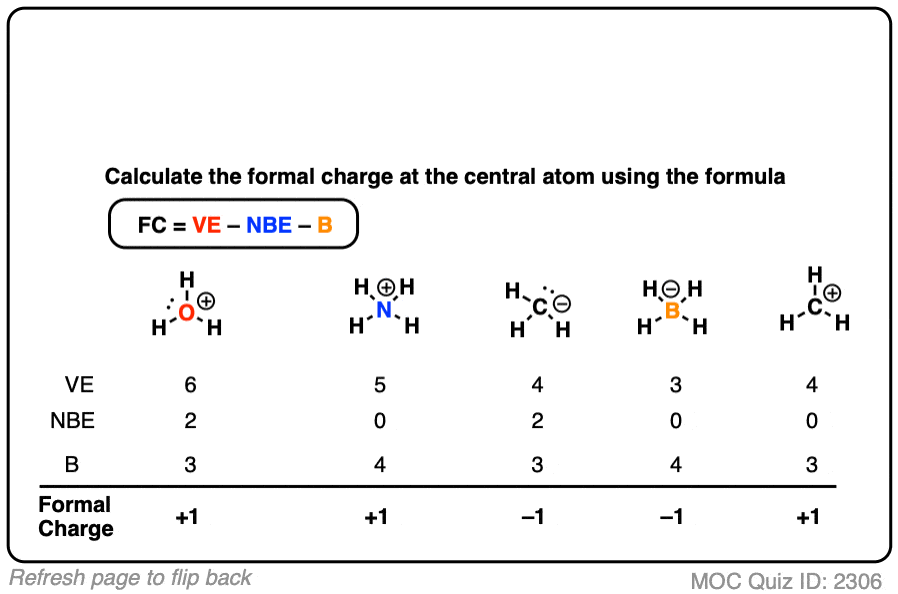
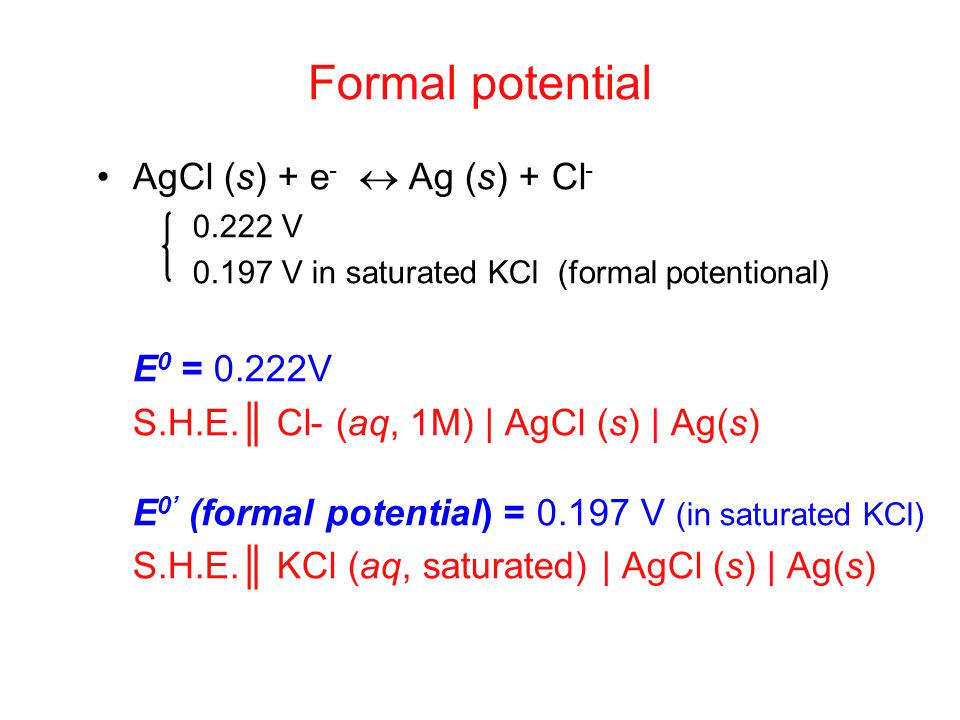
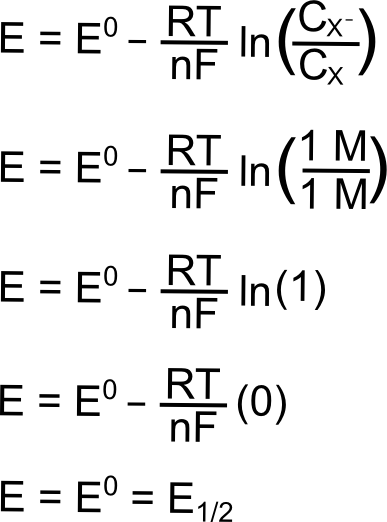Worked example: Calculating E° using standard reduction potentials | AP Chemistry | Khan Academy - YouTube

The standard electrode potential for the following reaction is `+1.33V`. What is the potential at ` - YouTube

Given standard electrode potentials, Fe^2 + 2e^-→ Fe, E^∘ = - 0.440 V Fe^3 + + 3e^-→ Fe, E^∘ = - 0.036 V The standard electrode potential (E^∘) for Fe^3 + + e^-→ Fe^2 + is:

Given electrode potentials are : Fe^3 + + e^- → Fe^2 + ; E^ = 0.771 V I2 + 2e^- → 2I^ ; E^ = 0.536 V Find the E^ cell for the cell reaction: 2Fe^3 + + 2I^ → 2Fe^2 + + I2 is :